Sensible, latent and total heat in evaporated water - steam - at different gauge pressures and boiling temperatures.

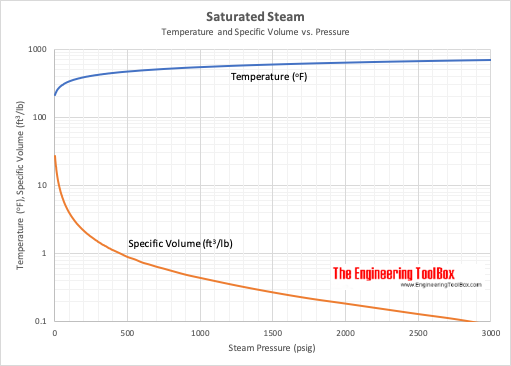
| Gauge Pressure (psig) | Temperature ( o F) | Specific Volume Saturated Vapor (ft 3 /lb) | Enthalpy | ||
|---|---|---|---|---|---|
| Saturated Liquid (Btu/lb) | Evaporated (Btu/lb) | Saturated Vapor (Btu/lb) | |||
| 25 (Inches Mercury Vacuum) | 134 | 142 | 102 | 1017 | 1119 |
| 20 (Inches Mercury Vacuum) | 162 | 73.9 | 129 | 1001 | 1130 |
| 15 (Inches Mercury Vacuum) | 179 | 51.3 | 147 | 990 | 1137 |
| 10 (Inches Mercury Vacuum) | 192 | 39.4 | 160 | 982 | 1142 |
| 5 (Inches Mercury Vacuum) | 203 | 31.8 | 171 | 976 | 1147 |
| 0 1) | 212 | 26.8 | 180 | 970 | 1150 |
| 1 | 215 | 25.2 | 183 | 968 | 1151 |
| 2 | 219 | 23.5 | 187 | 966 | 1153 |
| 3 | 222 | 22.3 | 190 | 964 | 1154 |
| 4 | 224 | 21.4 | 192 | 962 | 1154 |
| 5 | 227 | 20.1 | 195 | 960 | 1155 |
| 6 | 230 | 19.4 | 198 | 959 | 1157 |
| 7 | 232 | 18.7 | 200 | 957 | 1157 |
| 8 | 233 | 18.4 | 201 | 956 | 1157 |
| 9 | 237 | 17.1 | 205 | 954 | 1159 |
| 10 | 239 | 16.5 | 207 | 953 | 1160 |
| 12 | 244 | 15.3 | 212 | 949 | 1161 |
| 14 | 248 | 14.3 | 216 | 947 | 1163 |
| 16 | 252 | 13.4 | 220 | 944 | 1164 |
| 18 | 256 | 12.6 | 224 | 941 | 1165 |
| 20 | 259 | 11.9 | 227 | 939 | 1166 |
| 22 | 262 | 11.3 | 230 | 937 | 1167 |
| 24 | 265 | 10.8 | 233 | 934 | 1167 |
| 26 | 268 | 10.3 | 236 | 933 | 1169 |
| 28 | 271 | 9.85 | 239 | 930 | 1169 |
| 30 | 274 | 9.46 | 243 | 929 | 1172 |
| 32 | 277 | 9.1 | 246 | 927 | 1173 |
| 34 | 279 | 8.75 | 248 | 925 | 1173 |
| 36 | 282 | 8.42 | 251 | 923 | 1174 |
| 38 | 284 | 8.08 | 253 | 922 | 1175 |
| 40 | 286 | 7.82 | 256 | 920 | 1176 |
| 42 | 289 | 7.57 | 258 | 918 | 1176 |
| 44 | 291 | 7.31 | 260 | 917 | 1177 |
| 46 | 293 | 7.14 | 262 | 915 | 1177 |
| 48 | 295 | 6.94 | 264 | 914 | 1178 |
| 50 | 298 | 6.68 | 267 | 912 | 1179 |
| 55 | 300 | 6.27 | 271 | 909 | 1180 |
| 60 | 307 | 5.84 | 277 | 906 | 1183 |
| 65 | 312 | 5.49 | 282 | 901 | 1183 |
| 70 | 316 | 5.18 | 286 | 898 | 1184 |
| 75 | 320 | 4.91 | 290 | 895 | 1185 |
| 80 | 324 | 4.67 | 294 | 891 | 1185 |
| 85 | 328 | 4.44 | 298 | 889 | 1187 |
| 90 | 331 | 4.24 | 302 | 886 | 1188 |
| 95 | 335 | 4.05 | 305 | 883 | 1188 |
| 100 | 338 | 3.89 | 309 | 880 | 1189 |
| 105 | 341 | 3.74 | 312 | 878 | 1190 |
| 110 | 344 | 3.59 | 316 | 875 | 1191 |
| 115 | 347 | 3.46 | 319 | 873 | 1192 |
| 120 | 350 | 3.34 | 322 | 871 | 1193 |
| 125 | 353 | 3.23 | 325 | 868 | 1193 |
| 130 | 356 | 3.12 | 328 | 866 | 1194 |
| 135 | 358 | 3.02 | 330 | 864 | 1194 |
| 140 | 361 | 2.92 | 333 | 861 | 1194 |
| 145 | 363 | 2.84 | 336 | 859 | 1195 |
| 150 | 366 | 2.74 | 339 | 857 | 1196 |
| 155 | 368 | 2.68 | 341 | 855 | 1196 |
| 160 | 371 | 2.6 | 344 | 853 | 1197 |
| 165 | 373 | 2.54 | 346 | 851 | 1197 |
| 170 | 375 | 2.47 | 348 | 849 | 1197 |
| 175 | 377 | 2.41 | 351 | 847 | 1198 |
| 180 | 380 | 2.35 | 353 | 845 | 1198 |
| 185 | 382 | 2.29 | 355 | 843 | 1198 |
| 190 | 384 | 2.24 | 358 | 841 | 1199 |
| 195 | 386 | 2.19 | 360 | 839 | 1199 |
| 200 | 388 | 2.14 | 362 | 837 | 1199 |
| 205 | 390 | 2.09 | 364 | 836 | 1200 |
| 210 | 392 | 2.05 | 366 | 834 | 1200 |
| 215 | 394 | 2 | 368 | 832 | 1200 |
| 220 | 396 | 1.96 | 370 | 830 | 1200 |
| 225 | 397 | 1.92 | 372 | 828 | 1200 |
| 230 | 399 | 1.89 | 374 | 827 | 1201 |
| 235 | 401 | 1.85 | 376 | 825 | 1201 |
| 240 | 403 | 1.81 | 378 | 823 | 1201 |
| 245 | 404 | 1.78 | 380 | 822 | 1202 |
| 250 | 406 | 1.75 | 382 | 820 | 1202 |
| 255 | 408 | 1.72 | 383 | 819 | 1202 |
| 260 | 409 | 1.69 | 385 | 817 | 1202 |
| 265 | 411 | 1.66 | 387 | 815 | 1202 |
| 270 | 413 | 1.63 | 389 | 814 | 1203 |
| 275 | 414 | 1.6 | 391 | 812 | 1203 |
| 280 | 416 | 1.57 | 392 | 811 | 1203 |
| 285 | 417 | 1.55 | 394 | 809 | 1203 |
| 290 | 418 | 1.53 | 395 | 808 | 1203 |
| 295 | 420 | 1.49 | 397 | 806 | 1203 |
| 300 | 421 | 1.47 | 398 | 805 | 1203 |
| 305 | 423 | 1.45 | 400 | 803 | 1203 |
| 310 | 425 | 1.43 | 402 | 802 | 1204 |
| 315 | 426 | 1.41 | 404 | 800 | 1204 |
| 320 | 427 | 1.38 | 405 | 799 | 1204 |
| 325 | 429 | 1.36 | 407 | 797 | 1204 |
| 330 | 430 | 1.34 | 408 | 796 | 1204 |
| 335 | 432 | 1.33 | 410 | 794 | 1204 |
| 340 | 433 | 1.31 | 411 | 793 | 1204 |
| 345 | 434 | 1.29 | 413 | 791 | 1204 |
| 350 | 435 | 1.28 | 414 | 790 | 1204 |
| 355 | 437 | 1.26 | 416 | 789 | 1205 |
| 360 | 438 | 1.24 | 417 | 788 | 1205 |
| 365 | 440 | 1.22 | 419 | 786 | 1205 |
| 370 | 441 | 1.2 | 420 | 785 | 1205 |
| 375 | 442 | 1.19 | 421 | 784 | 1205 |
| 380 | 443 | 1.18 | 422 | 783 | 1205 |
| 385 | 445 | 1.16 | 424 | 781 | 1205 |
| 390 | 446 | 1.14 | 425 | 780 | 1205 |
| 395 | 447 | 1.13 | 427 | 778 | 1205 |
| 400 | 448 | 1.12 | 428 | 777 | 1205 |
| 450 | 460 | 1 | 439 | 766 | 1205 |
| 500 | 470 | 0.89 | 453 | 751 | 1204 |
| 550 | 479 | 0.82 | 464 | 740 | 1204 |
| 600 | 489 | 0.74 | 475 | 728 | 1203 |
| 650 | 497 | 0.69 | 483 | 719 | 1202 |
| 700 | 505 | 0.64 | 491 | 710 | 1201 |
| 750 | 513 | 0.6 | 504 | 696 | 1200 |
| 800 | 520 | 0.56 | 512 | 686 | 1198 |
| 900 | 534 | 0.49 | 529 | 666 | 1195 |
| 1000 | 546 | 0.44 | 544 | 647 | 1191 |
| 1250 | 574 | 0.34 | 580 | 600 | 1180 |
| 1500 | 597 | 0.27 | 610 | 557 | 1167 |
| 1750 | 618 | 0.22 | 642 | 509 | 1151 |
| 2000 | 636 | 0.19 | 672 | 462 | 1134 |
| 2250 | 654 | 0.16 | 701 | 413 | 1114 |
| 2500 | 669 | 0.13 | 733 | 358 | 1091 |
| 2750 | 683 | 0.11 | 764 | 295 | 1059 |
| 3000 | 696 | 0.08 | 804 | 213 | 1017 |
| 3206.2 2) | 705.40 | - | - | - | - |
1) Atmospheric pressure is used for the table except for 2)
2) Critical Point - At 3206.2 psia and 705.40 o F the vapor and liquid are indistinguishable. No change of state occurs when pressure increases above the critical point or when heat is added. At the critical point it is no longer referred to water or steam and it is not possible keep the water and steam apart.
NTP - Normal Temperature and Pressure - is defined as 20 o C (293.15 K, 68 o F) and 1 atm ( 101.325 kN/m 2 , 101.325 kPa, 14.7 psia, 0 psig, 30 in Hg, 760 torr)
At atmospheric pressure - 0 psig - water boils at 212 o F. 180 Btu/lb of energy is required to heat 1 lb of water to saturation temperature 212 o F.
Therefore, at 0 psig and 212 o F - the specific enthalpy of water is 180 Btu/lb.
Another 970 Btu/lb of energy is required to evaporate the 1 lb of water at 212 o F to steam at 212 o F. Therefore, at 0 psig - the specific enthalpy of evaporation is 970 Btu/lb.
The total specific enthalpy of the steam (or heat required to evaporate water to steam) at atmospheric pressure and 212 o F can be summarized to