
The once-daily DAYTRANA patch is a long-acting ADHD treatment that can be removed early to fit your child’s individual needs. †
† DAYTRANA should be applied 2 hours before an effect is needed. DAYTRANA should not be worn longer than 9 hours a day. Consult your doctor about early removal of the patch.




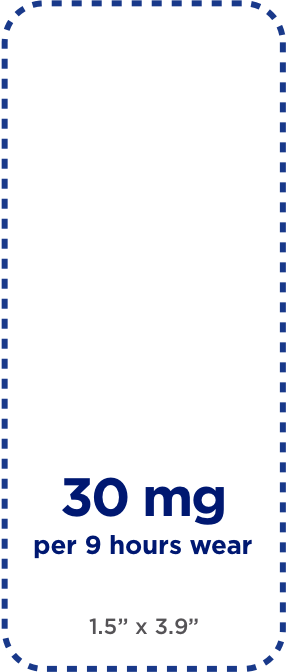
Sizes depicted are relative; dimensions appear below dosage strengths.

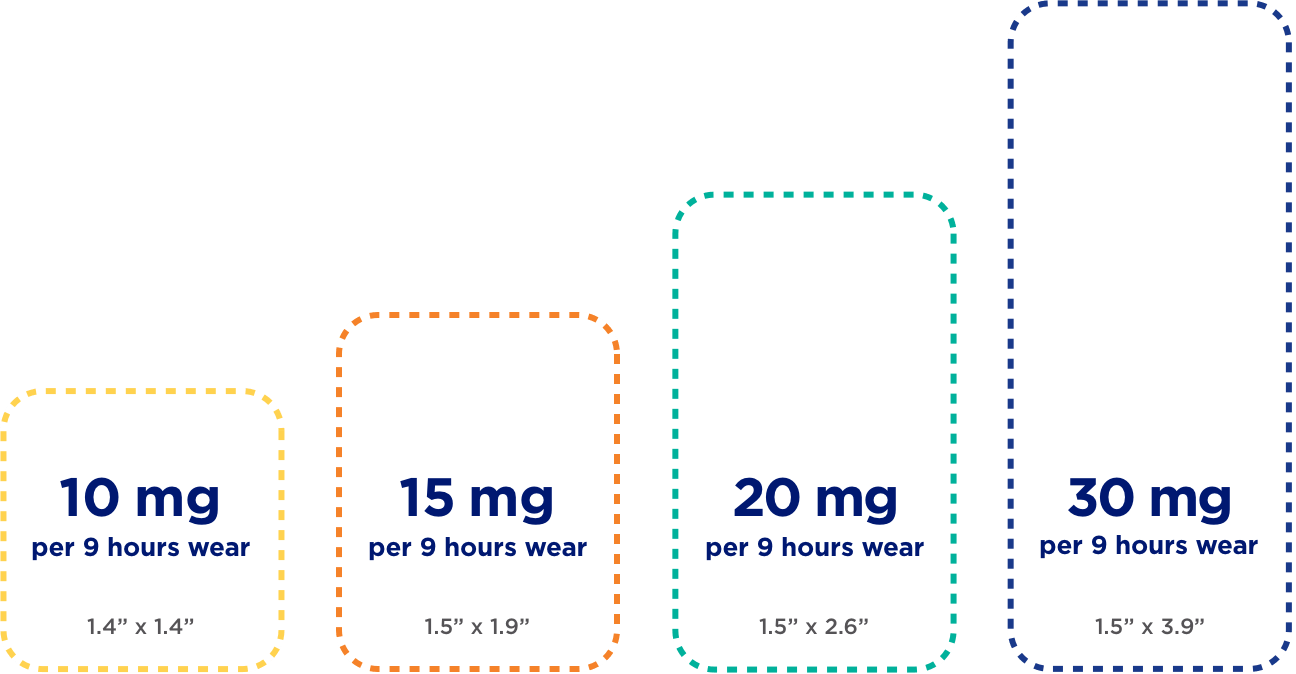
Sizes depicted are relative; dimensions appear below dosage strengths.
Each size delivers a different dosage strength designed to help manage the symptoms of ADHD in children ages 6-17. The suggested starting dose is 10 mg . The dosage may need to be gradually increased based on the patient's response.
Together, you and your doctor will find the right dose for your child. The patch provides up to 10 hours of efficacy when worn for up to 9 hours—the maximum recommended wear time.
Each size delivers a different dosage strength designed to help manage the symptoms of ADHD in children ages 6-17. The suggested starting dose is 10 mg . The dosage may need to be gradually increased based on the patient's response. Together, you and your doctor will find the right dose for your child. The patch provides up to 10 hours of efficacy when worn for up to 9 hours—the maximum recommended wear time.
NOTE: Because the medicine in the DAYTRANA patch does not have to be processed through the stomach, dosage strengths may be different from oral ADHD medications.

The layer that shows after you apply the patch
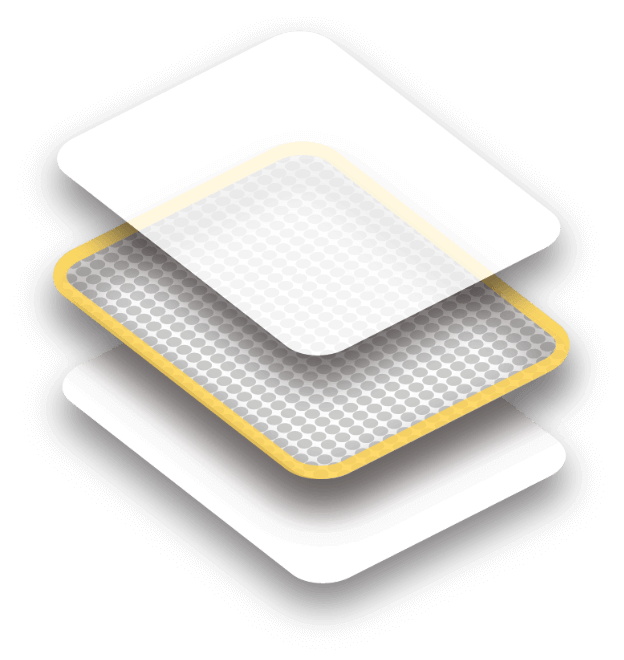
Sticks to the skin to deliver the medicine

Removed when you apply the patch

The layer that shows after you apply the patch
Sticks to the skin to deliver the medicine
Removed when you apply the patch
When applied correctly, the DAYTRANA patch should stay in place for the full duration of treatment. Exposure to water during bathing, swimming, or showering may affect how well the patch sticks, so be sure to follow the 6 steps below to ensure you apply the patch properly.

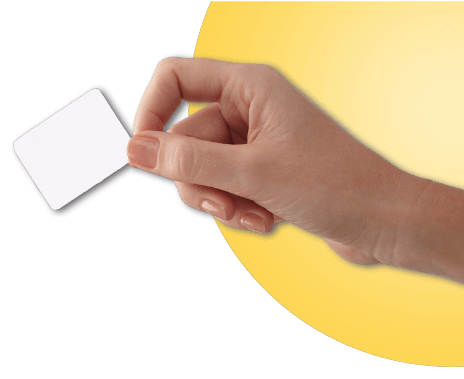
Carefully cut open the pouch containing the patch, and make sure it is not damaged. The patch should separate easily from the protective liner. Do not use patches that have been cut or damaged in any way. Throw away the patch if the protective liner is hard to remove.
Hold the patch with the protective liner facing you—the word "DAYTRANA" will appear backwards because you are looking at the bottom of the patch.
Gently bend the patch along the faint line, and slowly peel half the liner to expose the sticky surface underneath. Try to avoid touching the sticky part with your fingers. If you do touch it, wash your hands immediately after application.
Place the sticky side of the patch firmly on the hip and smooth it down. Make sure the skin is clean, dry, and cool without any powder, lotion, or oil. Alternate the hip on which the patch is worn each day to reduce potential irritation.
Gently fold back the other half and slowly peel off the remaining protective liner.
Press the entire patch firmly into place and hold for about 30 seconds. This ensures adhesion of the patch to the skin. Go over the edges with your fingers to make sure that it's secure.
Carefully cut open the pouch containing the patch, and make sure it is not damaged. The patch should separate easily from the protective liner. Do not use patches that have been cut or damaged in any way. Throw away the patch if the protective liner is hard to remove.
Hold the patch with the protective liner facing you—the word "DAYTRANA" will appear backwards because you are looking at the bottom of the patch.
Gently bend the patch along the faint line, and slowly peel half the liner to expose the sticky surface underneath. Try to avoid touching the sticky part with your fingers. If you do touch it, wash your hands immediately after application.
Place the sticky side of the patch firmly on the hip and smooth it down. Make sure the skin is clean, dry, and cool without any powder, lotion, or oil. Alternate the hip on which the patch is worn each day to reduce potential irritation.
Gently fold back the other half and slowly peel off the remaining protective liner.
Press the entire patch firmly into place and hold for about 30 seconds. This ensures adhesion of the patch to the skin. Go over the edges with your fingers to make sure that it's secure.
NOTE: Wash your hands immediately after applying the patch. Patches should not be reapplied with bandages, tape, or other household adhesives. Also, do not use hair dryers, heating pads, electric blankets, or other heat sources directly on the patch. If you have to replace a patch that has fallen off, the total wear time for the first and second patch should not be more than a total of 9 hours in 1 day. Do not reapply the same patch that fell off.

Peel the patch off slowly, then fold the used patch in half so that it sticks to itself.
Flush the used patch down the toilet or, if you have a septic tank, dispose of it in a lidded trash receptacle right away.
Remove any adhesive residue from the skin by gently rubbing the area with oil or lotion.
Peel the patch off slowly, then fold the used patch in half so that it sticks to itself.
Flush the used patch down the toilet or, if you have a septic tank, dispose of it in a lidded trash receptacle right away.
Remove any adhesive residue from the skin by gently rubbing the area with oil or lotion.
NOTE: Any unneeded or expired DAYTRANA patches should be disposed of properly. Remove patches from their protective pouches, peel off the liners, and fold the sticky sides together. Then flush the patches down the toilet or throw away in a lidded trash receptacle right away. Wash your hands after you handle the patch. Proper disposal is important since unneeded or expired patches still contain medication that could be harmful to small children or pets if touched or consumed.
Pay as little as $20 for each prescription of 30 patches if your out-of-pocket cost is $100 or less. If your out-of-pocket cost is more than $100, you will save a total of $80 on each prescription of 30 patches. Offer valid for up to a total of 12 prescriptions of 30 patches. Offer limited to one use per month. Offer limited to one per person and is not transferable. The parties reserve the right to change or end this program without notice at any time. See offer for full terms and conditions.
* TERMS AND CONDITIONS APPLY
This co-pay savings offer is only valid for commercially insured and cash-paying patients. This offer is not insurance. It is not valid for prescriptions covered by or submitted for reimbursement in whole or in part under Medicaid, Medicare, or other state or federal healthcare programs, including any state medical pharmacy assistance program.

DAYTRANA is a prescription central nervous system (brain) stimulant medicine used for the treatment of Attention-Deficit Hyperactivity Disorder (ADHD) in children and adolescents 6 to 17 years of age. DAYTRANA may help increase attention and decrease impulsive and hyperactive behavior in children with ADHD. It is not known if DAYTRANA is safe and effective in children under 6 years of age.
Abuse, misuse, and addiction: DAYTRANA is a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep DAYTRANA in a safe place to protect it from theft. Never give your DAYTRANA to anyone else because it may cause death or harm them. Selling or giving away DAYTRANA may harm others and is against the law. Tell your healthcare provider if your child has ever abused or been dependent on alcohol, prescription medicines, or street drugs.
DAYTRANA should not be used if your child is:
DAYTRANA may cause serious side effects including:
Before taking DAYTRANA, tell your HCP about all the medications your child takes and their medical conditions, including if they:
The most common side effects of DAYTRANA in children 6 to 12 years old include: Decreased appetite, trouble sleeping, nausea, vomiting, weight loss, tics; changes in mood, and trouble eating.
The most common side effects of DAYTRANA in children 13 to 17 years old include: Decreased appetite, nausea, trouble sleeping, weight loss, dizziness, stomach pain, and trouble eating.
DAYTRANA may also cause skin problems where it is applied (redness, small bumps, itching).
Please read Medication Guide and Full Prescribing Information including the Boxed Warning.
DAYTRANA ® and the Graphic Design are registered trademarks of Noven Therapeutics, LLC. For information call 1-877-567-7857 Please see our Online Privacy Policy for more information. © 2023 Noven Therapeutics, LLC. All rights reserved. For U.S. Audience Only. DAY-3516-16 12/2023
DAYTRANA is a prescription central nervous system (brain) stimulant medicine used for the treatment of Attention-Deficit Hyperactivity Disorder (ADHD) in children and adolescents 6 to 17 years of age. DAYTRANA may help increase attention and decrease impulsive and hyperactive behavior in children with ADHD. It is not known if DAYTRANA is safe and effective in children under 6 years of age.
Abuse, misuse, and addiction: DAYTRANA is a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep DAYTRANA in a safe place to protect it from theft. Never give your DAYTRANA to anyone else because it may cause death or harm them. Selling or giving away DAYTRANA may harm others and is against the law. Tell your healthcare provider if your child has ever abused or been dependent on alcohol, prescription medicines, or street drugs.
DAYTRANA should not be used if your child is:
DAYTRANA may cause serious side effects including:
Before taking DAYTRANA, tell your HCP about all the medications your child takes and their medical conditions, including if they:
The most common side effects of DAYTRANA in children 6 to 12 years old include: Decreased appetite, trouble sleeping, nausea, vomiting, weight loss, tics; changes in mood, and trouble eating.
The most common side effects of DAYTRANA in children 13 to 17 years old include: Decreased appetite, nausea, trouble sleeping, weight loss, dizziness, stomach pain, and trouble eating.
DAYTRANA may also cause skin problems where it is applied (redness, small bumps, itching).
Please read Medication Guide and Full Prescribing Information including the Boxed Warning.
Abuse, misuse, and addiction: DAYTRANA is a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep DAYTRANA in a safe place to protect it from theft. Never give your DAYTRANA to anyone else because it may cause death or harm them. Selling or giving away DAYTRANA may harm others and is against the law. Tell your healthcare provider if your child has ever abused or been dependent on alcohol, prescription medicines, or street drugs.
DAYTRANA should not be used if your child is:
DAYTRANA may cause serious side effects including:
Before taking DAYTRANA, tell your HCP about all the medications your child takes and their medical conditions, including if they:
The most common side effects of DAYTRANA in children 6 to 12 years old include: Decreased appetite, trouble sleeping, nausea, vomiting, weight loss, tics; changes in mood, and trouble eating.
The most common side effects of DAYTRANA in children 13 to 17 years old include: Decreased appetite, nausea, trouble sleeping, weight loss, dizziness, stomach pain, and trouble eating.
DAYTRANA may also cause skin problems where it is applied (redness, small bumps, itching).
Please be advised that Noven Therapeutics, LLC has no control over the content or presentation of the site you are about to view. Click the "Continue" button to proceed.
To return to daytrana.com click "Cancel," or close this window.
This site is intended for U.S. audiences only.
© 2022 Noven Therapeutics, LLC. All rights reserved.
Please be advised that Noven Therapeutics, LLC has no control over the content or presentation of the site you are about to view. Click the "Continue" button to proceed.
To return to daytrana.com click "Cancel," or close this window.
This site is intended for U.S. audiences only.
© 2022 Noven Therapeutics, LLC. All rights reserved.
Please be advised that Noven Therapeutics, LLC has no control over the content or presentation of the site you are about to view. Click the "Continue" button to proceed.
To return to daytrana.com click "Cancel," or close this window.
This site is intended for U.S. audiences only.
© 2022 Noven Therapeutics, LLC. All rights reserved.
Please be advised that Noven Therapeutics, LLC has no control over the content or presentation of the site you are about to view. Click the "Continue" button to proceed.
To return to daytrana.com click "Cancel," or close this window.
This site is intended for U.S. audiences only.
© 2022 Noven Therapeutics, LLC. All rights reserved.